The accumulation of aberrant proteins in the body will cause various neurodegenerative diseases. Amyloid β, one of these aberrant proteins, is a known risk factor for Alzheimer's disease. Eisuke Itakura, an assistant professor at Chiba University, says, "Human cells have functions for maintaining homeostasis. Scientists are now actively studying the typical intracellular protein degradation systems by autophagy and proteasome, but our knowledge of how cells act on aberrant external substances is still limited."
The research team led by Itakura gained new knowledge of the functions that human cells have for maintaining homeostasis through experiments in petri dishes. The team discovered a system in which cells could capture, degrade and remove aberrant extracellular proteins. This study will be published February 18th in the Journal of Cell Biology.
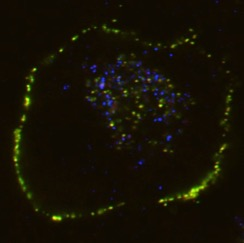
In these experiments, the team focused on Clusterin, an extracellular molecular chaperone. By developing an original proprietary cell internalization assay in which cells fluoresce when extracellular Clusterin is taken up, they established a new method for visually observing the state of proteolysis in the body (Fig 1).